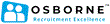Search Results: 27 vacancies
£ 47 - 52 per hour
...of Process systems; Centrifuge, Parts Washers, Autoclaves.
* An understanding of applicable regulations such as Annex 11, GAMP and 21 CFR Part 11.
* 7+ years’ experience in a GMP environment, preferably Pharma/Biopharma.
* Strong problem solving skills.
* Ability to...
£ 47 - 52 per hour
...ControlNet, DeviceNet, Profibus and OSIsoft PI Data Historian
* Knowledge of GAMP software development lifecycle, ANSI/ISA-S88 and S95 industry standards and 21 CFR Part 11
HOW TO APPLY
If you're interested in this role, click 'Apply Now' to forward an updated copy of your CV
€50k per annum
...Device manufacturing environment.
A working knowledge of quality systems such as ISO 13485 is essential. Experience and knowledge of 21 CFR Part 820 and EU GMP is an advantage.
Team player who can work effectively and proactively on cross-functional teams.
Experience/...
...including the building configuration qualification and validation of servers and PC s for use in a GxP environment according to GAMP 5 and CFR 21
Additionally the Lab IT Lead will assure full and continuous compliancy with regulatory and company requirements for example CSV...
£42.9k - £47.2k per annum
...to apply:
* Third Level Qualification Engineer or Designer with demonstrable understanding and application of Design Controls per 21 CFR 820.30 and Risk Management per ISO 14971:2019.
* 2-4+ years relevant experience is desirable but not essential.
* Demonstrable understanding...
...Life Cycle).
Good working knowledge of ISO13485 and ISO14971. IEC 62366 knowledge preferred (Usability).
Understanding of MDR and CFR requirements.
Prior experience with Jira/Structure/templated export or equivalent software tooling.
Additional Attributes:...
...Minimum of 4+ years' experience in Quality in a regulated environment.
Knowledge of the regulatory and compliance requirements of FDA 21 CFR Part 820 QSR, ISO 13485:2016, EU MDR.
Familiarity with SAP, Veeva Vault and Trackwise would be desirable.
Fastnet has a strict...
...understanding of quality management system principles (e.g., ISO 9001 or ISO 13485) and/or FDA design and development processes to 21 CFR part 820.
~ At least one year experience in electrical and/or mechanical verification (Design Verification Testing/ DVT), including the...
...lifecycle and support the local teams in term analysis
Leakage investigation: support teams in implementing and conducting OFR and CFR and participate actively in the investigations into important suspected cases of leakage to understand the root cause and develop corrective...
...Bachelor's degree in Computer Science, Engineering, or a related field.
*
Strong knowledge of regulatory guidelines, such as FDA 21 CFR Part 11, EU Annex 11, and GAMP 5.
*
Experience in computer system validation within a regulated industry, preferably...
...Life Cycle)
~ Good working knowledge of ISO13485 and ISO14971
~ IEC 62366 knowledge preferred (Usability)
~ Understanding of MDR and CFR requirements
~ Prior experience with Jira/Structure/templated export or equivalent software tooling
~ Strong communication skills...
...Bachelor's degree in Computer Science, Engineering, or a related field.
*
Strong knowledge of regulatory guidelines, such as FDA 21 CFR Part 11, EU Annex 11, and GAMP 5.
*
Experience in computer system validation within a regulated industry, preferably...
€50k per annum
...environment.
Preferred Knowledge, Skills and Abilities:
A thorough working knowledge of quality systems such as ISO 13485, 21 CFR Part 820 and/or EU GMP is essential. Knowledge of 21 CFR Part 210 / 211 would be considered an advantage.
Must have excellent communication...
...understanding of quality management system principles (e.g., ISO 9001 or ISO 13485) and/or FDA design and development processes to 21 CFR part 820
~1+ year experience in electrical and/or mechanical verification (Design Verification Testing/ DVT), including the drafting...
...Bachelor's degree in Computer Science, Engineering, or a related field.
· Strong knowledge of regulatory guidelines, such as FDA 21 CFR Part 11, EU Annex 11, and GAMP 5.
· Experience in computer system validation within a regulated industry, preferably pharmaceuticals...
...medical device or combination products in a regulated environment.
Knowledge of the regulatory and compliance requirements of FDA 21 CFR Part 820 QSR and CFR Part 4, ISO 13485, EU MDR and Risk Management Standard for Medical Devices ISO 14971 2019.
Familiarity with SAP...
...Lead design review, site acceptance and installation of equipment
- Write, Review and Approve CQV documents following established 21 CFR standards, both internally and externally. Document requests could include: Standard Operating Procedures, Impact Assessments, Risk Assessments...
...role is responsible for a key support activity on site supporting the Quality Management system processes within an ISO 13485 & FDA 21 CFR Part 820 regulatory environment.
Core Responsibilities:
• Responsible for technical inspection of electro-mechanical parts & instrumentation...
...• Design of FPGA systems for radio communication systems including digital front-end DSP such as filters, up and down-conversion, DPD, CFR.
• Active collaboration with a multidisciplinary team on architecture and design with digital design expertise. Provide inputs to architecture...
...role is responsible for a key support activity on site supporting supplier quality management services within an ISO 13485 and FDA 21 CFR Part 820 regulatory environment.
Core Responsibilities
• To provide day to day inspection expertise in the areas of Component and...