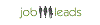Average salary: €34,000 /yearly
More statsGet new jobs by email
- ...supervision and guidance of ISL technical and operational teams. Like all SGS personnel, they are committed to upholding stringent technical and GMP standards, ensuring the safety of medications for the multitude of patients relying on the products we test. Due to growth, we have...SuggestedLocal areaWorldwideFlexible hours
- ...following: Working on the daily operation and maintenance of a wide variety of HVAC, clean and grey utilities equipment in a modern GMP facility Diagnosing and resolving problems with utility systems and equipment Conducting routine inspections and preventative maintenance...SuggestedPermanent employmentLocal areaShift workNight shiftDay shift
- ...communications skills. ~ Good written and verbal communication skills for both technical and non-technical audiences. ~ Knowledge of GMP, regulatory requirements, computer system validation. Preferable: ~7 years + as a controls engineer in a highly automated...Suggested
- ...communications skills. ~ Good written and verbal communication skills for both technical and non-technical audiences. ~ Knowledge of GMP, regulatory requirements, computer system validation. ~ Preferable ~ Process Engineering experience. ~ Experience in DCS batch...Suggested
- ...site, and the successful candidate will help to build the organization, the facility and the culture to enable a successful startup of GMP operations. The QC Systems and Compliance team provides support to the QC function owning cross lab programs such as electronic systems...SuggestedLong term contract
- ...leadership and expertise for automation support of manufacturing operations in accordance with appropriate Good Manufacturing Practices (GMP) and safety guidelines. This leadership role includes mentoring Process Automation Engineers on process control system configuration,...SuggestedFor contractorsWorking Monday to Friday
- ...: Bulk API, sterile products, secondary packaging, Devices and to a lesser extent lab projects. ~ Experience in the following areas: GMP manufacturing, LEAN, Automation, construction, construction quality, project controls, the engineering disciplines, safety, scheduling....SuggestedTemporary workFor contractorsFor subcontractorLong term contractLong distanceFlexible hours
- ...worksheets, methods and reports (tabular, trend and labels) within the QCL electronic systems. # Supports the Authoring/review of key GMP documents such as GMP standard operating procedures, training materials, test methods creation documents, local qualification documents...SuggestedLocal area
- ...with at least 2 years experience managing a team. Manager level requires you must have 8+ years experience within pharmaceutical/biotech GMP production facility and at least 3+ years leading teams. #REGNIELSM #LI-Onsite #IRELIM #JOBSIEST Does this sound like you? Apply...SuggestedPermanent employmentLocal area
- ...the team’s area of responsibility. Previous supervisory experience desirable Ideally a minimum of 5 years experience within a GLP/GMP analytical laboratory environment within the pharmaceutical or medical device industry. Excellent organisational skills are required...Suggested
- ...Talent Development, Manufacturing Training with a focus on regulated environments. Strong understanding of Good Manufacturing Practices (GMP), regulatory requirements, and compliance training. Proven leadership experience in managing and developing L&D teams at site level....SuggestedLocal areaShift work
£ 43.16 - 51.79 per hour
..., oversee vendor testing, and analyse data. Lead design reviews and recommend optimal sterilisation procedures. Ensure compliance with GMP practices and regulatory standards. Support regulatory documentation and submission processes. The start date is for ASAP. The initial...SuggestedHourly payContract workWork at officeImmediate startRemote work3 days week1 day week- ...pharmaceutical products (preferably DeltaV) They have demonstrated technical leadership and teamwork abilities. Experience in GMP, regulatory requirements, computer system validation, and cybersecurity. Good written and verbal communication skills for both technical...Suggested
- ...SME) level experience with PLC and HIM systems. Proven experience in designing, implementing, and maintaining HMI and SCADA systems. GMP environment experience (Pharma, Biotech or Medical Devices manufacturing) is a must and only candidates with this experience can and will...SuggestedPermanent employmentContract workRelocation
- ...Standards Performing equipment cleaning, preparation and execution as well as completing associated documentation Adhering to safety and GMP (Good Manufacturing Practice) requirements at all times when carrying out tasks Performing various tests and in-process sampling...SuggestedPermanent employmentLocal areaShift work
- ...customer. Qualifications Degree in relevant scientific discipline. Familiarity with pharmaceutical and/or laboratory operations (GMP, Documentation, Autoclaves, Aseptic Technique) Preferred 6 months laboratory/GMP experience. Good planning and organisational...Full timePart timeImmediate startShift work
- ...associated Computer System maintenance activities Key requirements include: Several years within a relevant QA CSV position within a GMP environment Major Capex project experience highly desirable Experience implementing Computer System Quality guidelines and...Contract workLocal area
- ...Proven experience working closely with project managers. Knowledge of medical device quality standards ISO13485/FDA practices, EUMDR, GMP and similar regulated industry standards. Strong statistical understanding and experience e.g. DOE and/or Gage R&R. Strong...
- ...and interpersonal communication skills with the ability to effectively interact with a broad spectrum of audiences You bring strong GMP knowledge To be considered for this positon you must hold a BS/BA in Life sciences and 2+ years related industry experience. #REGNIEQA...Temporary workLocal area
- ...recurring E&I issues Assisting in development, implementation and updates to SOPs, policies, plans, and programs Representing the GMP Calibration Program in all audit and regulatory inspections Vendor & Budget Management: Evaluating and managing contracts...For contractorsLong term contractLocal area
- ...pharmaceutical products (preferably DeltaV) They have demonstrated technical leadership and teamwork abilities. Experience in GMP, regulatory requirements, computer system validation, and cybersecurity. Good written and verbal communication skills for both technical...
- ...solving skills and a hands-on approach to managing operational challenges. Familiarity with ISO 13485 and Good Manufacturing Practices (GMP) is a distinct advantage. Immediate interviews available for suitable candidates. Note: By applying for this position, you...Permanent employmentFull timeImmediate start
- ...to individual team member performance. Ensure ongoing continuous improvement and future planning. Establish and Maintain safety, GMP and environmental Standards. Identify and implement changes to work practices in conjunction with appropriate team members. Ensure...Long term contractLocal area
- ..., supports and trains other quality team members. • Monitor and drive compliance within the development process and/or operations to GMP and the requirements of the QMS within assigned area. • Trouble shooting quality issues identified during product development and commercialisation...
- ...honours degree qualified, ideally with 10 years relevant industry experience including leadership roles ~ Proven capability within a GMP Manufacturing environment. Key Attributes : High performance in delivery of work. High learning agility and flexibility and...Local area
- ...below, but not all. Key Objectives/Deliverables: Operations Support: Ensure all activities are conducted following all HSE and GMP requirements. Work closely with the Leadership to coordinate necessary resources to support activities throughout the duration of...
- ...supporting documentation, SOPs, PFMEA, Control Plans and process work instructions compliant with current Good Manufacturing Practices (GMP) Transferring and implementing processes, either from development, or from another manufacturing facility. Own budgetary,...Fixed term contract
- ...automation discipline, with a minimum of 5 years’ experience in industry, preferably in a pharmaceutical, biotechnology, blue chip or relevant GMP manufacturing environment. The ideal candidate will have excellent communication skills and strong problem skills. The candidate...Permanent employmentFor contractorsRemote work
- ...Filtration Skids and Clean Utilities an advantage Self-motivated, proactive approach with a commitment to meet deadlines Knowledge of GMP'S, regulatory requirements, equipment commissioning and qualification Good written and verbal communication skills with both...For contractors
- ...scientific progress for product(s) of responsibility in alignment with Annex 16 requirements. Communication and education of personnel in GMP requirements and regulations. Quality Monitoring Programs Monitoring of GMP compliance and verification of the effective...Full timeLocal area